Drug Optimization: Current Research

Discovering and evaluating the factors that influence how therapeutic agents work
The primary focus of the Division of Pharmacotherapy and Experimental Therapeutics(DPET) is the optimization of drug therapy through the discovery and evaluation of key factors that influence how therapeutic agents work in patients. Our faculty advance clinical practice by leading innovative clinical and translational research and by educating and training clinical scientists and current practitioners.
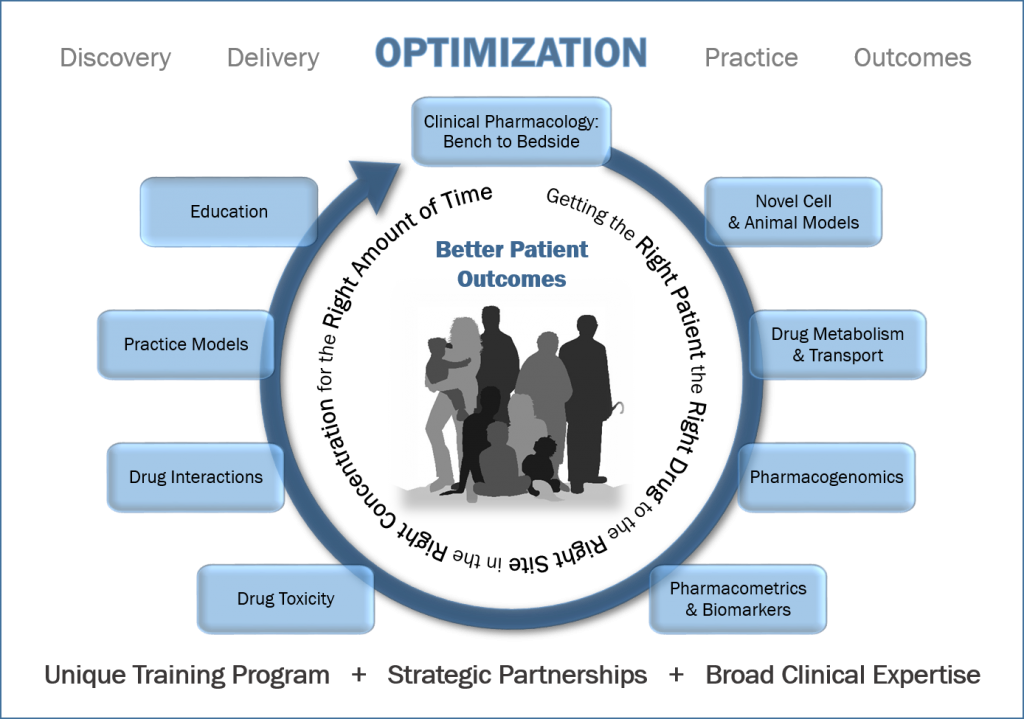 Our faculty lead research programs that aim to translate experimental and clinical pharmacology discoveries into precision medicine and apply these discoveries to improve patient care. DPET is focused on collaborative clinical and translational research projects, with a particular emphasis on developing world-class research programs in quantitative pharmacology, pharmacogenomics and precision medicine, drug safety, and experimental therapeutics. DPET faculty also lead innovative educational research projects focused on the scholarship of teaching and learning.
Our faculty lead research programs that aim to translate experimental and clinical pharmacology discoveries into precision medicine and apply these discoveries to improve patient care. DPET is focused on collaborative clinical and translational research projects, with a particular emphasis on developing world-class research programs in quantitative pharmacology, pharmacogenomics and precision medicine, drug safety, and experimental therapeutics. DPET faculty also lead innovative educational research projects focused on the scholarship of teaching and learning.
Our graduate and postdoctoral training programs develop scientists who excel at conducting innovative and clinically relevant translational research that integrates biomedical and pharmaceutical sciences through use of laboratory-based and computational modeling approaches that leverage preclinical and clinical investigations.
Our research projects span several therapeutic areas:
- Infectious diseases
- Oncology/hematology
- Special populations
- Cardiology
- Neurology
- Hepatology/gastroenterology/transplant
- Pulmonology

Derek Bartlett
Dr. Bartlett’s research aims to improve the translatability of preclinical studies and to unlock the potential of functional precision medicine through in vitro/ex vivo drug testing and combination therapy optimization.

Kim Brouwer
Dr. Brouwer directs an NIH-funded research program focused on hepatobiliary drug disposition and development and refinement of in vitro model systems to predict in vivo hepatobiliary disposition, drug interactions, and hepatotoxicity.

Yanguang Cao
Dr. Cao’s group is interested in developing system pharmacology platforms (models) integrating PK/PD to facilitate drug development and optimize therapeutics for cancers and autoimmune diseases.

Amber Cipriani
Dr. Cipriani’s appointment is cofunded by UNC Hospitals, where she serves as a clinical oncology specialist in thoracic oncology.

Amanda Corbett
Dr. Corbett’s expertise are in HIV, antiviral, and opportunistic infection clinical pharmacy and ethnopharmacology.

Mackenzie Cottrell
Dr. Cottrell’s research focuses on describing pharmacokinetic
/pharmacodynamic relationships in mucosal tissues for antiretrovirals being used in HIV prevention and cure interventions.

Daniel Crona
Dr. Crona’s translational research program focuses on how genetic variations can lead to differences in the pharmacokinetics and pharmacodynamics of therapeutic treatments used in oncology, and how inter-individual differences in clinical pharmacology measures can affect survival and drug toxicity phenotypes.

Julie Dumond
Dr. Dumond is currently conducting a clinical study in aging, HIV-infected subjects to explore the effects of cellular aging and frailty on antiretroviral toxicity and efficacy.

Bob Dupuis
Dr. Dupuis has expertise in clinical pharmacology, drug metabolism, adverse effects, outcomes, and enhancement of care in solid organ transplantation.

Muluneh Fashe
Dr. Fashe’s research goal is to develop and validate in vitro experimental models that can be used to provide mechanistic explanations for altered xenobiotic metabolism and clearance and optimize the safe and effective use of therapeutics in patients.

Amber Frick
Dr. Frick’s responsibilities at the School are to develop an expertise in the implementation and assessment of new approaches to and best practices in teaching while also taking part in collaborative teaching activities.

Erin Heinzen
Dr. Heinzen focuses on the genetic and genomic basis of epilepsy disorders, including analyses of the role of germline mutations, somatic mutations, and how regulation of the cellular transcriptome influences the risk and presentation of seizures.

Klarissa Jackson
Dr. Jackson’s research interests focus on drug metabolism and toxicology to better understand the mechanisms and risk factors of adverse drug reactions and improve drug safety.
Angela Kashuba
Dr. Kashuba’s research focuses on the clinical pharmacology of drugs used in the treatment, prevention, and cure of HIV infection.

Craig Lee
Dr. Lee’s research focuses on cytochrome P450 metabolism, cardiovascular experimental therapeutics, and precision medicine/pharmacogenomics.

Dulcie Lai
Dr. Lai’s research focuses on modeling SLC35A2 epilepsy in hiPSC-derived neuronal models.

Benyam Muluneh
Dr. Muluneh’s research endeavors include developing strategies to improve medication adherence in clinical practice, optimizing tolerability and safety of chemotherapy in cancer patients, and promoting chemotherapy access and affordability to underinsured and uninsured patients with cancer in the United Sates and beyond.

Adam Persky
Dr. Persky’s research focuses on translating the science of learning and memory into practical application in the classroom and experiential settings.

Jo Ellen Rodgers
Dr. Rodgers maintains an active clinical practice in the outpatient Cardio-Oncology Clinic with UNC Health Care and her primary research interests are in the care of heart failure and cardio-oncology patients.

Elias Rosen
Dr. Rosen’s research focuses on the development of methods to measure intracellular distribution of therapeutics and their metabolites in a variety of biological matrices using mass spectrometry imaging.

Zhenwei Song
Dr. Song’s research is focused on developing safer and more efficient AAV vectors for liver-target gene therapy.

Deborah Sturpe
Dr. Sturpe’s research focuses on the scholarship of teaching and learning. In particular, she has interest in curricular development and competency-based assessment.

Patricia Termini
Patricia Termini is the Director of the Master of Professional Science (MPS) in Regulatory Science and aims to support a new generation of regulatory professionals in developing the knowledge and skills to be successful in bringing new medicines to patients.

Jacqueline B. Tiley
Dr. Tiley’s research interest is on disease- and drug-mediated alterations in hepatic and placental transport proteins and its impact on drug disposition and toxicity.

Paul Watkins
Dr. Watkins focuses on developing innovative approaches to investigate the underlying mechanisms of rare or “idiosyncratic” toxicities that are often not discovered until late in clinical development.

Dennis Williams
Dr. Williams is an associate professor in the Division of Pharmacotherapy and Experimental Therapeutics.

Tim Wiltshire
Dr. Wilshire’s research focuses on taking the pharmacogenetic knowledge we already have and develop approaches for that information to be used effectively in clinical practice.
William Zamboni
Dr. Zamboni’s research interests focus on the application of pharmacokinetic, pharmacodynamic, and pharmacogenetic principles in the optimization of the chemotherapeutic treatment of cancer.
 In the fall of 2023, the U.S. Food and Drug Administration announced an award up to $50 million over five years to the University of North Carolina at Chapel Hill and Duke University to establish the Research Triangle Center of Excellence in Regulatory Science and Innovation (CERSI). The center will also involve collaborations with North Carolina State University and North Carolina Central University, a leading historically black university.
In the fall of 2023, the U.S. Food and Drug Administration announced an award up to $50 million over five years to the University of North Carolina at Chapel Hill and Duke University to establish the Research Triangle Center of Excellence in Regulatory Science and Innovation (CERSI). The center will also involve collaborations with North Carolina State University and North Carolina Central University, a leading historically black university.
Triangle CERSI, the newest of only five CERSIs across the country, will work with FDA scientists to perform cutting edge scientific research to better inform and support the FDA’s needs. The four other FDA-funded CERSIs include the University of Maryland, the University of California at San Francisco in partnership with Stanford University, Johns Hopkins University, and Yale University in partnership with Mayo Clinic.
Paul Watkins, M.D., Howard Q. Ferguson Distinguished Professor of Pharmacy, will be the corresponding principal investigator of the award. The Triangle CERSI will establish many collaborations between university investigators and scientists at the FDA to conduct research projects that will fin in gaps in knowledge required to enable the FDA to make the best regulatory decisions and to craft the best guidance documents and policies that have broad implications for public health. The Center will also serve as a hub for training and education of students at all levels in the rapidly evolving field of regulatory science.
A primary focus of DPET is the optimization of drug therapy and precision medicine. Given the expertise contained within DPET, we have built a precision dosing initiative into our strategic plan. This initiative is based on the premise that standard doses of many drugs evaluated in Phase III studies do not meet the needs of all patients, and lead to poor response or unnecessary side effects. We strive to improve patient outcomes and lower healthcare costs by transforming how medication dosing is selected and optimized at the time of prescribing. This premise is not new – therapeutic drug monitoring that targets patient variability has been used for decades for certain drugs, and pharmacists are adept at providing this monitoring. However, with the widespread implementation of the electronic medical record, and the expansion of pharmacometrics into modeling appropriate dosing for special populations, we believe initial dosing strategies could be better optimized at the time the prescription is written.
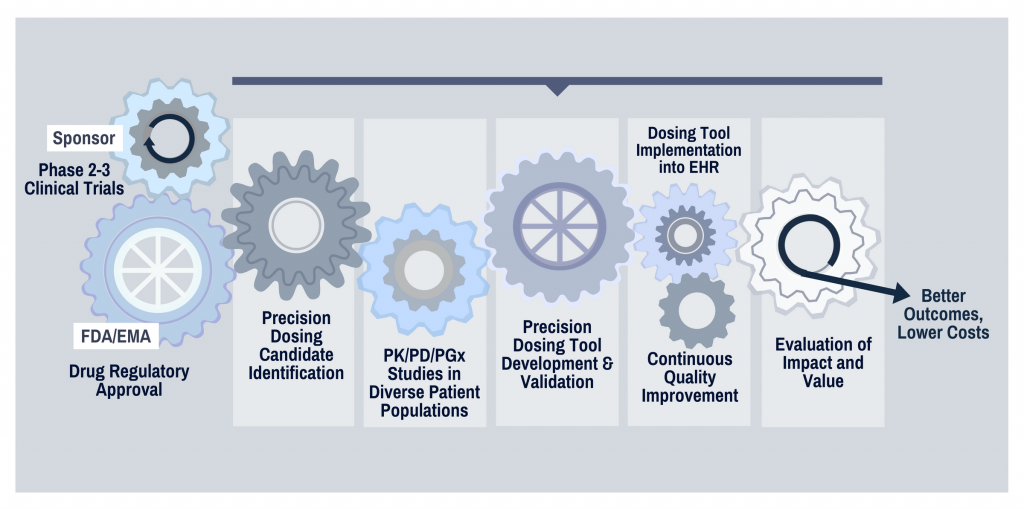 Certain drug classes, such as anticoagulants, antiinfectives, anticancer agents, and opioids are ideal candidates for individualized dosing due to their common use in high-risk patient populations, narrow therapeutic window, and substantial variability in response between patients.
Certain drug classes, such as anticoagulants, antiinfectives, anticancer agents, and opioids are ideal candidates for individualized dosing due to their common use in high-risk patient populations, narrow therapeutic window, and substantial variability in response between patients.
DPET has established a multidisciplinary research network that converges expertise in clinical and quantitative pharmacology, data science, medicine, implementation science, and innovative clinical practice models to transform how medication dosing is optimized in individual patients.
Researchers in DPET occupy approximately 75,000 square feet of laboratory space in Genetic Medicine Building and Kerr Hall on the health sciences campus at UNC Chapel Hill.
These research facilities are adjacent to the UNC School of Medicine, UNC School of Nursing, UNC Gillings School of Global Public Health, UNC Adams School of Dentistry, and UNC Health Medical Center, allowing DPET researchers to collaborate closely with colleagues across disciplines and giving DPET faculty and trainees a strong presence in the heart of Carolina’s health sciences campus.
The labs in Genetic Medicine Building and Kerr Hall contain seating areas for lab personnel, lab bench areas, fume hood rooms, tissue culture rooms, equipment rooms and cold rooms. Both buildings also contain a vivarium for vertebrate animal research, and provide space for shared equipment, microscopy rooms, additional freezer storage, chemical storage, and meeting rooms.
Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Laboratory
The purpose of the Clinical Pharmacology and Analytical Chemistry Laboratory (Core E) is to provide a centralized unit to facilitate the pharmacological and analytical studies of HIV/AIDS related clinical, translational, and basic science research at UNC-Chapel Hill and at our collaborating institutions, and to establish collaboration with AIDS investigators at other national and international institutions.
Advanced Translational Pharmacology and Analytical Chemistry (ATPAC) Lab
The ATPAC core lab consists of the Analytical Chemistry and Pharmacology Core (ACPC) lab and the Translational Oncology and Nanoparticle Drug Development Initiative (TONDDI) lab. The core lab uses analytical chemistry and pharmacologic infrastructure to support the translational development of drugs, anticancer agents, carrier-mediated agents and biologics.
The Pharmacometrics Core assists investigators at each step in the pharmacokinetic/pharmacodynamic research process, from study design and data analysis to publication.
Graduate Program
Our PhD program develops scientists who excel at conducting innovative and clinically relevant translational research that integrates biomedical and pharmaceutical sciences in a laboratory- and computer-based environments that leverage preclinical and clinical investigations. Details regarding the PhD program can be found here.
Postdoctoral Fellowships
We offer a variety of fellowships sponsored by the NIH, pharmaceutical industry partners, and traditional academic-funded fellowships. Details regarding these fellowships can be found here.
We are partnering across the campus, state, nation and around the world to advance research and advance knowledge and treatment for patients to discover solutions for the world’s most challenging health issues.

UNC Health Sciences Campus
The School is part of the University of North Carolina at Chapel Hill, a major research university with a large teaching hospital and schools of medicine, public health, nursing, and dentistry.
- Ranked 5th nationally for federal research among all universities
- $1.1 billion in sponsored research from all sources annually
- Eleventh largest research university in research volume and annual expenditures

Eshelman Innovation
Eshelman Innovation is a health care innovation engine that bridges the inspiration of academia and the spirit of entrepreneurship to turn groundbreaking ideas into real products and services. They are here to catalyze ideas for the greater good. Since its creation in 2014, Eshelman Innovation has expanded well beyond the walls of the school of pharmacy at UNC-Chapel Hill. Eshelman Innovation works with faculty across the state and collaborators around the world. Their work brings together academic firepower alongside funders and donors to find creative solutions to major health care problems.

Research Triangle Park
UNC anchors one corner of North Carolina’s famed Research Triangle Park, which hosts an abundance of pharmaceutical, biotech, and health-care companies. This environment offers many opportunities for collaboration in research, education, and patient care with partners in academia, industry, and health care.

Pinnacle Hill
The University of North Carolina at Chapel Hill and Deerfield Management have entered into a partnership to create Pinnacle Hill, a company seeking to discover new medicines to address the significant unmet medical needs of our times. Deerfield will invest up to $65 million of targeted funding through Pinnacle Hill, as well as providing significant drug development expertise to advance promising therapeutic research at UNC-Chapel Hill. Learn more here.

PharmAlliance
PharmAlliance is a unique international partnership between three global leaders in pharmacy education, the University of North Carolina at Chapel Hill, Monash University, and University College London. PharmAlliance partners work collaboratively to inspire and train tomorrow’s professional leaders and practitioners to transform education delivery and address major research challenges in pharmacy and the pharmaceutical sciences.

Structural Genomics Consortium
The SGC (Structural Genomics Consortium) is a not-for-profit, public-private partnership that performs basic science of relevance to drug discovery. Research is conducted at several sites around the world to produce reagents, proteins, antibodies, assays, and data that support exploration of the human genome. All material and intellectual output of the SGC is placed in the public domain for use without restriction. The first SGC laboratory to operate in the USA is located in the UNC Eshelman School of Pharmacy. Learn more here.



