Katelyn Arnold is a Research Assistant Professor in the Division of Chemical Biology and Medicinal Chemistry (CBMC). Dr. Arnold’s research interest is in therapeutic development of synthetic heparin. She uses chemoenzymatically synthetized oligosaccharides in various animal models to investigate the relationship between oligosaccharide structure and function to understand anti-inflammatory mechanisms. This work also involves pharmacokinetic studies using methods and standards specific for synthetic heparin.
Innovations and Transformations in Pharmacy and Pharmaceutical Sciences
About
ITPS is a three-week course offered to students from around the world. This program will expose students to world-class professors from the No.1-ranked school of pharmacy in the United States. Discover cutting-edge research and innovative technologies as professors explore the lifecycle of drugs and vaccines in our modern world.
Students will receive 4 UNC credit hours and an official UNC transcript upon successful completion of the program.
Deadline to apply is 8 March 2024. Apply now to save your spot for this unique summer learning experience!
Can’t join us in-person in North Carolina? We also have a virtual option available! Click on the “Virtual Program” tab to learn more.
“Through the study of this program, I not only have a deeper understanding of various fields of pharmacy, but also a strong guidance for the direction of subsequent study. I also learned a lot of cutting-edge knowledge in the field of pharmacy, including the establishment and use of various models. During this process, I have seen the world’s cutting-edge research results and advanced scientific research ideas, and my participation in the [ITPS Program] has provided guidance for my future study and research.”
– ITPS 2023 Participant
Informational Session
Join us for an informational session to learn more about the ITPS program and connect with the program faculty and coordinators.
23 February 2024 | 8 a.m. EDT
https://unc.zoom.us/j/98022451616?pwd=TGpQMjRhUGZaQWRyNUE0Z2VlNlo0Zz09
Passcode: UNCITPS
Questions about ITPS?
Sarah Merritt, PharmD, MPH
Global Program Education Coordinator
+1-919-918-1799
 Program Components
Program Components
Presentations: Presentations will take students through the lifespan of the drug, from drug discovery to drug delivery, experimental therapeutics, outcomes and policy, then clinical use. We will start with some foundational knowledge, then move into individual research to highlight the innovations currently happening in the field. Each week there will be several presentations dedicated to professional development, such as CV and personal statement writing, critical evaluation of literature, grant writing, etc.
Site Visits: Each week we will have one or two site visits to local pharmacies or pharmaceutical companies. Students will get to visit a variety of sites to explore the many areas of pharmacy and pharmaceutical sciences, such as startup companies, large manufacturing companies, and hospitals.
Skills Labs: Each week we will have one or two skills labs that will give students time to explore hands-on activities such as pharmacy compounding, or learn new skills such as excel proficiency or HPLC simulations.
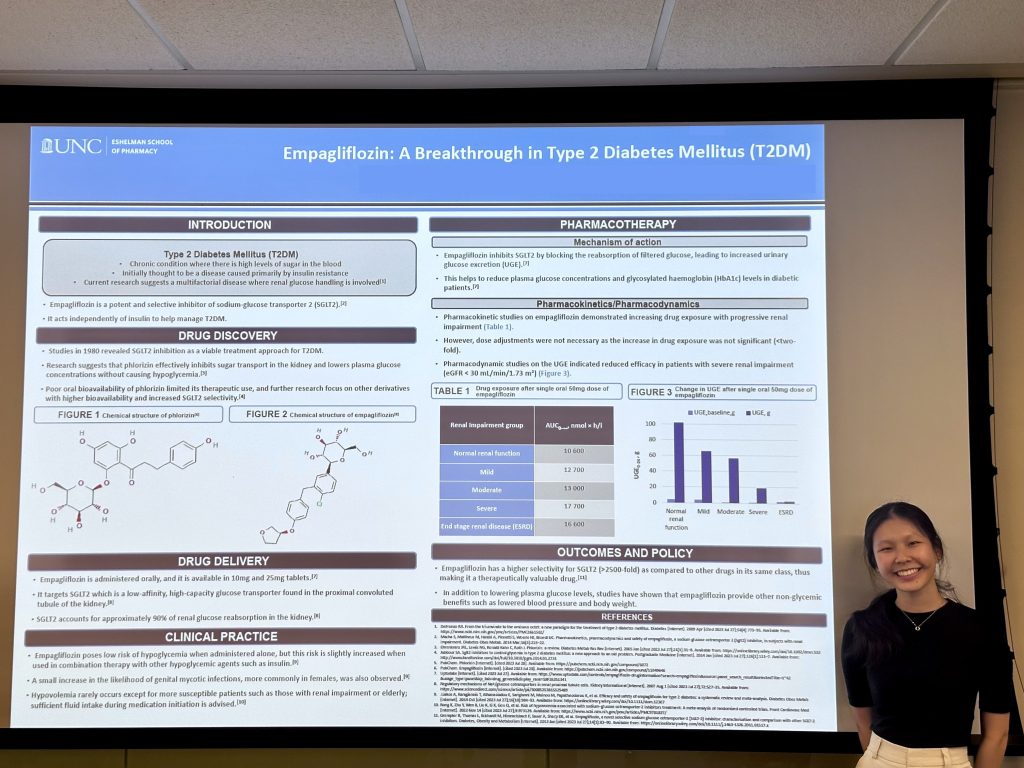
Final Project: Students will apply what they have learned in a final project where they will follow a drug through its lifespan. Students will research a drug, write a scientific abstract, then create a poster to present during their final day, simulating the steps of presenting at a scientific conference.
Cultural Activities: Get to know the culture of North Carolina through various cultural activities. We will introduce students to local cuisine, such as NC barbeque, take them to favorite sporting events, and provide opportunities to interact with UNC students.
Daily Program Schedule (Monday through Friday):
8:30-11:30: Presentations
11:30-13:00: Lunch break
13:00-16:00: Site visit, skills lab, or cultural activity
Meet some of the instructors:
Kim L. R. Brouwer
Kim L.R. Brouwer, Pharm.D., Ph.D., is the William R. Kenan Jr. Distinguished Professor in the Division of Pharmacotherapy and Experimental Therapeutics of the UNC Eshelman School of Pharmacy, associate dean for research and graduate education, and a professor in the curriculum in toxicology.
Yanguang Cao
Yanguang Cao, Ph.D., joined the UNC Eshelman School of Pharmacy initially as an assistant professor and then promoted as an associate professor in the division of Pharmacotherapy and Experimental Therapeutics. Prior to joining the School, Cao served as a research assistant professor at SUNY Buffalo for two years after completing a postdoctoral training program at SUNY, Buffalo.
Stephen Eckel
Stephen Eckel, Pharm.D., M.H.A., is associate dean for global engagement and a associate professor in the Division of Practice Advancement and Clinical Education. He leads a two-year Master of Science in Pharmaceutical Sciences with a specialization in health-system pharmacy administration. This degree is hosted at eight hospitals, five of which are in North Carolina and others are located in 3 other states. At UNC Medical Center, he is director of pharmacy for innovation services, where he is residency program director for the 2-year health system pharmacy administration and leadership residency program. He has worked with almost 200 residents over the years.
Michael Bruce Jarstfer
Michael Jarstfer, PhD, is an Associate Professor within the Division of Chemical Biology and Medicinal Chemistry and the Associate Dean for Graduate Education. He is also the Director of Graduate Studies for the Pharmaceutical Sciences PhD program. Dr. Jarstfer has expertise in drug target identification, high-throughput-screening, medicinal chemistry, and compound optimization for drug discovery as well as pharmacology in preclinical animal models.
Yutian Ma, PhD
Yutian Ma is a Postdoctoral Research Associate at the Division of Pharmacoengineering and Molecular Pharmaceutics at the UNC Eshelman School of Pharmacy. He conducted his Ph.D. at the University of Melbourne (Australia). He has a background in biomedical engineering, biomolecular engineering, nanomedicine and drug delivery. In Australia, he worked on developing nanoengineered drug delivery systems for the treatment of inner ear disease. He is currently working on developing non-viral vectors for improving the efficacy and safety of RNA therapeutics.
Sachiko Ozawa
Sachiko Ozawa, Ph.D., M.H.S., is a health economist whose work focuses on generating evidence that can be used to improve the health of populations globally. Her research focuses on examining the value of vaccines, assessing the economic burden of diseases and examining the demand and utilization of health care. She is interested in the interface between pharmacy and public health from the perspective of a global health economist.
Megan Roberts
Megan Roberts, PhD, is an assistant professor in the Division of Pharmaceutical Outcomes and Policy and the Director of Implementation Science in Precision Health and Society at the UNC Eshelman School of Pharmacy. Her research focuses on evaluating and improving the implementation of genomic medicine. In particular, she is interested in implementation research aimed at improving quality of care and reducing racial disparities in cancer prevention and treatment.
Program Fee
ITPS 2024 Program fee: $5,750 USD
Program fee includes the following:
- All program/administrative fees (including visa invitation)
- Coursework preparation and delivery
- Tuition (four credit hours)
- On-campus housing
- Food (breakfast, lunch, dinner)
- International health insurance
- Transportation for program activities
- Materials for skills labs
- Cultural activities
*Flights and visa fees are not included
Program payment deadline: 15 June 2024
Discounts:
Discounts are available for early registration (on or before 31 January 2024) and for group discounts. Contact globalpharmacy@unc.edu for more information.
On-Campus Housing
Students will receive on-campus housing included in their program fee. Experience life as a UNC student by staying in the UNC dorms within easy walking distance to the UNC Eshelman School of Pharmacy and downtown Chapel Hill.
Virtual Program
Can’t join us in in-person? We also offer a virtual program!
What is included in the virtual program?
Students will get access to all the presentations to join live or watch recorded at their convenience. We will also offer a weekly recitation exclusively for virtual participants to review program material and interact with UNC faculty and students. Upon successful completion of the program, students will receive a Certificate of Completion.
Deadline to apply is 21 June 2024. Apply now to save your spot for this unique summer learning experience!
Virtual Program Fee: $500 USD
Discounts: Discounts are available for early registration (on or before 30 April 2024) and for group discounts. Contact globalpharmacy@unc.edu for more information.
Frequently Asked Questions
Where is the UNC Eshelman School of Pharmacy located?
UNC Eshelman School of Pharmacy is located in Chapel Hill, North Carolina, USA. Chapel Hill is a college town, with easy access to North Carolina’s state capital of Raleigh, as well as Research Triangle Park, which is the largest research park in the United States. Located midway between New York City and Miami, Chapel Hill is only a 2.5-hour drive to either the mountains to the west, or the beach to the east.
Who should apply to this program?
This program is appropriate for learners in undergraduate programs, graduate programs, or working professionals. It will introduce you to core concepts in pharmacy and pharmaceutical sciences, then expand on them by discussing innovative research currently happening in the field.
What is the process for applying?
Complete your initial application and deposit ($250 USD) at this link. You will then get sent an email with the full application to complete. Once you submit this application, it will begin your enrollment into UNC as a study abroad student. Once your application has been processed, you will be able to set up your UNC login and email and request your visa form (Form I-20). If you have any questions throughout this process, please reach out to us at globalpharmacy@unc.edu.
What is the difference between the residential and virtual programs?
The residential program is held at the UNC Eshelman School of Pharmacy in Chapel Hill, North Carolina. It includes presentations, site visits, skills labs, and cultural activities. The virtual program is held via zoom and can be completed from anywhere throughout the world. The virtual program includes the presentations and a weekly recitation. At the end of the residential program, students receive four credit hours and an official transcript that can be used to transfer credit back to their home institution if allowed, whereas in the virtual program students receive a certificate of completion.
What opportunities will I have to interact with UNC students and faculty?
Each presentation is taught by a different individual, which provides great opportunity to network and meet many (up to 30) UNC faculty, postdocs, and students! Additionally, UNC students are engaged in the skills labs, site visits, and cultural activities, providing lots of opportunity to make connections.