Angela Kashuba Lab
Angela Kashuba Lab
Welcome to the UNC School of Pharmacy Clinical Pharmacology and Analytical Chemistry Laboratory web site! Our faculty and staff facilitate research in preclinical and clinical pharmacology and analytical chemistry in pursuit of a common goal: optimizing the prevention and treatment of HIV infection. We invite you to learn more about our vigorous research agenda. You are welcome to contact us for further information on our activities and services. We also encourage you to visit the UNC Center for AIDS Research website and our Clinical Pharmacology and Analytical Chemistry Core website.
Current Research Projects
The Kashuba lab is engaged in a number of research projects. Current studies include the following sponsored research:
- Ronald Swanstrom, Principal Investigator; Kashuba ADM, Core Director; Cottrell M, Investigator. UNC Center for AIDS Research Core E: Clinical Pharmacology/Analytical Chemistry.The Clinical Pharmacology/Analytical Chemistry Core of the UNC CFAR is designed to provide a centralized facility to advise, support and perform pharmacological and analytical studies of AIDS related clinical and basic science research projects. Projects range from in-vitro correlations of drug disposition, drug interactions and drug toxicity to clinical pharmacokinetic-dynamic evaluations of interactions, efficacy, toxicity and the development of drug resistance. NIAID P30-AI50410-20; 08/01/11 – 07/31/21; $206,195.
- Buse, Principal Investigator; Kashuba ADM, Mentor. North Carolina Translational & Clinical Sciences Institute (NC TraCS). This grant provides 5 years of support for UNC’s CTSA award, the NC Translational and Clinical Sciences (TraCS) Institute. Dr. Kashuba serves within the Education Program of the NC TraCS Institute and mentors KL2 Scholars. NIH 5UL1TR001111-05; 05/01/2016- 04/30/2018; salary support.
- Kashuba ADM, Principal Investigator.Multi-Species Mechanisms of Drug Bio-distribution in HIV Tissue Reservoirs. This project aims to identify what species differences and pharmacologic barriers exist in extracellular and intracellular antiretroviral biodistribution and efficacy in eliminating active HIV reservoirs. NIH/NIAID 1R01AI111891-04 3/15/2014-2/28/2019 $519,821.
- Boggess, Principal Investigator; Kashuba ADM, Research Director. UNC Building Interdisciplinary Research Careers in Women’s Health (K12). Kashuba will serve as Associate Research Director and Resource Laboratory Network Director for this K12 project. NIH 2K12HD001441-18; 9/30/2015 – 7/31/2020; $35,550.
- Kashuba ADM, Principal Investigator. Novel Mass Spectrometry Imaging Methods to Quantify Antiretroviral Adherence. It is important to identify if someone is taking a medication regularly and as prescribed to optimize their health and wellbeing. Sometimes, patients have trouble remembering if and when they miss doses; other times, even though patients are taking their medication, it is not getting into the body in the right amount. Quickly monitoring medications in 5-10 hair strands using our novel imaging technology called IR-MALDESI will allow patients and their doctors to see how much medication they are exposed to over 1 or more months, and help identify challenges to taking medication in both research and clinical settings. This proposal will optimize and explore the acceptability and feasibility of using IR-MALDESI for monitoring medications in hair. NIH/NIAID; 01/01/2016-12/31/2020; $711,764.
- Margolis, Principal Investigator; Kashuba ADM,Project Leader; Cottrell M, Investigator. Collaboratory of AIDS Researchers for Eradication (CARE). Including scientists from leading universities and Merck Research Laboratories, Qura Therapeutics, and Macrogenics, the Collaboratory of AIDS Researchers for Eradication (CARE) will seek to eradicate HIV infection by developing and testing therapies that will permanently destroy the viral reservoir. NIH 1UM1AI126619-02; 7/14/2016 – 6/30/2021; $66,750.
- Lewin, Principal Investigator; Kashuba ADM, Consortium PI. Long term persistence of HIV in the liver and the clinical impact on HIV-HBV co-infection. One of the main challenges in the management of HIV is that it has long lived forms that persist in the face of antiviral therapy. Several studies suggest that the liver may be a reservoir of HIV even on long term ART. To determine whether HIV persistence in the liver on ART is a consequence of sub therapeutic levels of ARV in liver, as previously described for lymphoid tissue and rectal tissue, we propose to measure ARV levels in human liver, plasma and PBMC samples both pre and following at least 2 years ART by both LC-MS/MS and IR-MALDESI IMS methods. NHMRC/University of Melbourne 1101836IPF 16-4433; 11/28/2016 – 12/31/2019; $32,805.
- Cottrell M, Principal Investigator. Animal Model for Testing SIV Latency Reversal Strategies. The UNC Clinical Pharmacology and Analytical Chemistry Laboratory will develop and validate Romedepsin and prostratin analytical methods in plasma and tissues for the overall project. Furthermore, therapeutic dosing strategies will be designed and developed for these compounds and perform pharmacokinetic analysis, modeling and simulation using the resulting concentration data. SubAward 0047867(126557-1)/5R01AI119346 (Apetrei); 8/1/2015-1/31/2019; $50,888/4 years.
- Cottrell M, Principal Investigator. Differential HIV infection and Tenofovir activity in pre- and post-menopausal women. The UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Laboratory will be providing tenofovir and tenofovir diphosphate concentration analysis on surgery-derived tissue explants and clinical biopsy samples. SubAward S120282-3/4R01HD072705-05 (Doncel); 6/1/2016-5/31/2018; $43,815.
- Cottrell M, Principal Investigator. Intramuscularly Administered TMB 607 in HIV Negative Volunteers. This project will develop an analytical method for determination of concentration of TMB-607 in human plasma. TaiMed Biologics, Inc. Contract AGR#A16-1466-001; 5/26/2016-5/25/2019; $22,646.
- Cottrell M, Principal Investigator. Pharmacokinetics Testing for CONRAD A15-137, titled Exploratory Pharmacokinetic and Pharmacodynamic Study of Oral F/TAF for the Prevention of HIV UNC laboratory will perform pharmacokinetic (PK) analysis on de-identified samples collected under CONRAD A15-137 study with UNC for the purpose of sample analysis and PK analysis including the provision of input into the statistical analysis plan, manuscript and other appropriate PK analyses reports. USAID SubAward MAPS1-17-073/AID-OAA-A-14-00011 (MAPS1); 3/15/2017-4/14/2018; $605,946.
- Cottrell M, Principal Investigator. Chimerix CMX048 Feasibility_Work Order#2. UNC laboratory will perform Optimization IR-MALDESI assay for a single compound, CMX16792 (CMX8521-PPP), in gut tissue and assessment of lower limit of quantitation that can be achieved for the analysis of CMX16792 in gut tissues. Chimerix, Inc., Award PO#WP100215; 6/28/2017-12/27/2017; $4,893.
- Cottrell M, Principal Investigator. Work Order 3_Evaluation of sulfolane and 3-nitrobenzyl alcohol on signal for CMX048 and Evaluation of sensitivity and linearity of CMX048 in PBMC lysate. UNC laboratory will perform the work of this project. Chimerix, Inc., Award PO#WP100405; 10/02/2017-4/01/2018; $7,466.
- Cottrell M, Investigator. The University of North Carolina for AIDS Research. The UNC CFAR is notable for the breadth of research activity, the high level of excellence of it member researchers, and the importance of its domestic and international partners in fighting the HIV epidemic. NIH; 2P30AI050410-19; 8/1/2016–7/31/2021; $7,466.
- Cottrell M, Investigator. Collaboratory of AIDS Researchers for Eradication. The Collaboratory of AIDS Researchers for Eradication (CARE) will seek to eradicate HIV infection by developing and testing therapies that will permanently destroy the viral reservoir. NIH/NIAI; 1UM1AI126619-01; 7/14/2016-6/30/2021; $18,356.
- Cottrell M, Project Lead. Qura Project 1F Using a Novel Hollow Fiber Model System to Predict the In Vivo Pharmacodynamics of Latency Reversing Agents. One of the most advanced investigational strategies to eradicate HIV has been termed “Kick and Kill”. The “Kick” refers to using latency reversing agents (LRAs) to induce viral transcription from latently infected cells. In this study, we apply a model of HIV latency to the hollow fiber culture system, and determine if it can reproduce the clinical trial results of daily and intermittent LRA dosing. In achieving the aims of this project, we will establish proof of concept that this novel hollow fiber model of HIV latency can predict in vivo pharmacodynamics for LRAs. Qura Therapeutics, LLC; 1/01/2017 – 12/31/2017; $82,519.
- Wira, Principal Investigator; Cottrell M, Consortium PI. Chemical Contraceptive Control of Microbicides in the Female Reproductive Tract. Dr. Kashuba’s lab will pharmacokinetic analysis expertise and utilize bioanalytical methods developed for parent and intracellular active metabolites in tissue and cellular matrices in the female genital tract. NIH/Dartmouth College, 1R01AI117739-02; 2/01/2015 -1/31/2018; $64,096.
Recent Publications
Long-acting rilpivirine (RPV LA) pre-exposure prophylaxis does not inhibit vaginal transmission of RPV-resistant HIV-1 nor select for high frequency drug resistance in humanized mice. Melody K, Roy CN, Kline C, Cottrell ML, Evans D, Shutt K, Pennings PS, Keele BF, Bility M, Kashuba ADM, Ambrose Z. J Virol. 2020 Jan 22. pii: JVI.01912-19. doi: 10.1128/JVI.01912-19. [Epub ahead of print] PubMed PMID: 31969438
Using Real-Time Adherence Feedback to Enhance Communication About Adherence to Antiretroviral Therapy: Patient and Clinician Perspectives. Hill LM, Golin CE, Pack A, Carda-Auten J, Wallace DD, Cherkur S, Farel CE, Rosen EP, Gandhi M, Asher Prince HM, Kashuba ADM. J Assoc Nurses AIDS Care. 2020 Jan-Feb;31(1):25-34. doi: 10.1097/JNC.0000000000000089. PubMed PMID: 31033629; PubMed Central PMCID: PMC6815236
Antiretroviral Penetration across Three Preclinical Animal Models and Humans in Eight Putative HIV Viral Reservoirs. Devanathan AS, Pirone JR, Akkina R, Remling-Mulder L, Luciw P, Adamson L, Garcia JV, Kovarova M, White NR, Schauer AP, Blake K, Sykes C, Burgunder EM, Srinivas N, Rosen EP, Kashuba ADM. Antimicrob Agents Chemother. 2019 Dec 20;64(1). pii: e01639-19. doi:10.1128/AAC.01639-19. Print 2019 Dec 20. PubMed PMID: 31611355
Delayed gastrointestinal-associated lymphoid tissue reconstitution in duodenum compared with rectum in HIV-infected patients initiating antiretroviral therapy. Sainz T, Serrano-Villar S, Mann S, Ma ZM, Utay NS, Thompson CG, Chun TW, Kashuba AD, Siewe B, Albanese A, Troia-Cancio P, Sinclair E, Somasunderam A, Yotter T, Moreno S, Pollard RB, Landay A, Miller CJ, Asmuth DM. AIDS. 2019 Dec 1;33(15):2289-2298. doi: 10.1097/QAD.0000000000002361. PubMed PMID: 31764094; PubMed Central PMCID: PMC6905119
Decreased Tenofovir Diphosphate Concentrations in a Transgender Female Cohort: Implications for Human Immunodeficiency Virus Preexposure Prophylaxis. Cottrell ML, Prince HMA, Schauer AP, Sykes C, Maffuid K, Poliseno A, Chun TW, Huiting E, Stanczyk FZ, Peery AF, Dellon ES, Adams JL, Gay C, Kashuba ADM. Clin Infect Dis. 2019 Nov 27;69(12):2201-2204. doi: 10.1093/cid/ciz290. PubMed PMID: 30963179
A Translational Approach to Predicting the Efficacy of Maraviroc-based Regimens as HIV Pre-exposure Prophylaxis. Srinivas N, Cottrell M, Maffuid K, Prince HA, Nelson JAE, White N, Sykes C, Dellon ES, Madanick RD, Shaheen NJ, Gonzalez D, Kashuba ADM. Antimicrob Agents Chemother. 2019 Nov 18. pii: AAC.01729-19. doi: 10.1128/AAC.01729-19. [Epub ahead of print] PubMed PMID: 31740561
Food insecurity is associated with lower levels of antiretroviral drug concentrations in hair among a cohort of women living with HIV in the United States. Leddy AM, Sheira LA, Tamraz B, Sykes C, Kashuba ADM, Wilson TE, Adedimeji A,
Merenstein D, Cohen MH, Wentz EL, Adimora AA, Ofotokun I, Metsch LR, Turan JM, Bacchetti P, Weiser SD. Clin Infect Dis. 2019 Oct 14. pii: ciz1007. doi: 10.1093/cid/ciz1007
Human Immunodeficiency Virus Type 1 RNA Detected in the Central Nervous System (CNS) After Years of Suppressive Antiretroviral Therapy Can Originate from a Replicating CNS Reservoir or Clonally Expanded Cells. Joseph SB, Kincer LP, Bowman NM, Evans C, Vinikoor MJ, Lippincott CK, Gisslén M, Spudich S, Menezes P, Robertson K, Archin N, Kashuba A, Eron JJ, Price RW, Swanstrom R. Clin Infect Dis. 2019 Sep 27;69(8):1345-1352. doi: 10.1093/cid/ciy1066. PubMed PMID: 30561541
Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Benhabbour SR, Kovarova M, Jones C, Copeland DJ, Shrivastava R, Swanson MD, Sykes C, Ho PT, Cottrell ML, Sridharan A, Fix SM, Thayer O, Long JM, Hazuda DJ, Dayton PA, Mumper RJ, Kashuba ADM, Victor Garcia J. Nat Commun. 2019 Sep 20;10(1):4324. doi: 10.1038/s41467-019-12141-5. PMID:31541085
Infrared Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging of Human Hair to Characterize Longitudinal Profiles of the Antiretroviral Maraviroc for Adherence Monitoring. Gilliland WM Jr, Prince HMA, Poliseno A, Kashuba ADM, Rosen EP. Anal Chem. 2019 Aug 20;91(16):10816-10822. doi: 10.1021/acs.analchem.9b02464. Epub 2019 Aug 6. PubMed PMID: 31345022
Association between Use of Methadone, Other Central Nervous System Depressants, and QTc Interval-Prolonging Medications and Risk of Mortality in a Large Cohort of Women Living with or at Risk for Human Immunodeficiency Virus Infection. Tamraz B, Reisner L, French AL, King ST, Fischl MA, Ofotokun I, Kashuba A, Milam J, Murphy K, Augenbraun M, Liu C, Finley PR, Aouizerat B, Cocohoba J, Gange S, Bacchetti P, Greenblatt RM. Pharmacotherapy. 2019 Sep;39(9):899-911. doi:10.1002/phar.2312. Epub 2019 Aug 13. PubMed PMID: 31332819
HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim WK, Knapp PE, Kashuba ADM, Hauser KF, McRae M. J Neurovirol. 2019 Aug;25(4):560-577. doi: 10.1007/s13365-019-00757-8. Epub 2019 May 17. PubMed PMID: 31102185; PubMed Central PMCID: PMC6750988
Heterogeneous antiretroviral drug distribution and HIV/SHIV detection in the gut of three species. Thompson CG, Rosen EP, Prince HAM, White N, Sykes C, de la Cruz G, Mathews M, Deleage C, Estes JD, Charlins P, Mulder L, Lovarova M, Adamson L, Arora S, Dellon ES, Peery AF, Shaheen NJ, Gay C, Muddiman DC, Akkina R, Garcia JV, Luciw P, Kashuba ADM. Sci Transl Med. 2019 Jul 3;11(499). pii: eaap8758. doi:10.1126/scitranslmed.aap8758. PMID:31270274
Antiretroviral drug concentrations in lymph nodes: a cross-species comparison of the effect of drug transporter expression, viral infection, and sex in humanized mice, nonhuman primates, and humans. Burgunder E, Fallon JK, White N, Schauer A, Sykes C, Remling-Mulder L, Kovarova M, Adamson L, Luciw P, Garcia JV, Akkina R, Smith PC, Kashuba ADM. J Pharmacol Exp Ther. 2019 Sep;370(3):360-368. doi:10.1124/jpet.119.259150. Epub 2019 Jun 24.
Seminal Tenofovir Concentrations, Viral Suppression, and Semen Quality With Tenofovir Alafenamide, Compared With Tenofovir Disoproxil Fumarate (Spanish HIV/AIDS Research Network, PreEC/RIS 40). Imaz A, Niubó J, Cottrell ML, Perez E, Kashuba ADM, Tiraboschi JM, Morenilla S, Garcia B, Podzamczer D. Clin Infect Dis. 2019 Sep 27;69(8):1403-1409. doi: 10.1093/cid/ciy1074. PubMed PMID: 30561517; PubMed Central PMCID: PMC6763637
Patient and clinician perspectives on optimizing graphical displays of longitudinal medication adherence data. Pack AP, Golin CE, Hill LM, Carda-Auten J, Wallace DD, Cherkur S, Farel CE, Rosen EP, Gandhi M, Asher Prince HM, Kashuba ADM. Patient Educ Couns. 2019 Jun;102(6):1090-1097. doi: 10.1016/j.pec.2018.12.029. Epub 2019 Jan 2. PubMed PMID: 30626550; PubMed Central PMCID: PMC6525638
Contemporary Drug-Drug Interactions in HIV Treatment. Devanathan AS, Anderson DJC, Cottrell ML, Burgunder EM, Saunders AC, Kashuba ADM. Clin Pharmacol Ther. 2019 Jun;105(6):1362-1377; PMID: 30739315
Predicting efavirenz concentrations in the brain tissue of HIV-infected individuals and exploring their relationship to neurocognitive impairment. Srinivas N, Joseph SB, Robertson K, Kincer LP, Menezes P, Adamson L, Schauer AP, Blake KH, White N, Sykes C, Luciw P, Eron JJ, Forrest A, Price R, Spudich S, Swanstrom R, Kashuba AD. Clin Transl Sci. 2019 May;12(3):302-311. doi: 10.1111/cts.12620. Epub 2019 Feb 27.
Estimating human immunodeficiency virus (HIV) prevention effects in low-incidence settings. Rudolph JE, Cole SR, Eron JJ, Kashuba AD, Adimora AA. Epidemiology. 2019 May;30(3):358-364 PMID: 30640216
Prescription of Antibacterial Drugs for HIV-Exposed, Uninfected Infants, Malawi, 2004-2010. Ewing AC, Davis NL, Kayira D, Hosseinipour MC, van der Horst C, Jamieson DJ, Kourtis AP; Breastfeeding, Antiretrovirals and Nutrition study team. Emerg Infect Dis. 2019 Jan;25(1). doi: 10.3201/eid2501.180782. PMID: 30561313
Disparate effects of cytotoxic chemotherapy on the antiviral activity of antiretroviral therapy: implications for treatments of HIV-infected cancer patients. Medina-Moreno S, Zapata JC, Cottrell ML, Le NM, Tao S, Bryant J, Sausville E, Schinazi RF, Kashuba AD, Redfield RR, Heredia A. Antivir Ther. 2019;24(3):177-186. doi: 10.3851/IMP3285. PubMed PMID: 30574873; PubMed Central PMCID: PMC6779049
Effect of Hormonal Contraception on Pharmacokinetics of Vaginal Tenofovir in Healthy Women: Increased Tenofovir Diphosphate in Injectable Depot Medroxyprogesterone Acetate Users. Thurman AR, Schwartz JL, Brache V, Chen BA, Chandra N, Kashuba ADM, Weiner DH, Mauck C, Doncel GF. J Acquir Immune Defic Syndr. 2019 Jan 1;80(1):79-88. doi: 10.1097/QAI.0000000000001864. PMID: 30212395
CPAC VALIDATES NOVEL ASSAY TO MEASURE ANTIRETROVIRALS IN DRIED BLOOD SPOT FOR ADHERENCE MONITORING
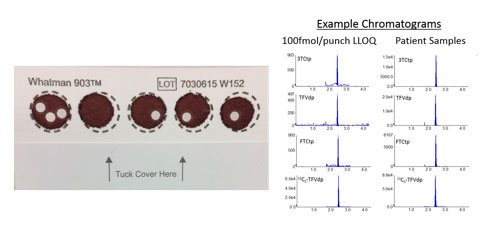
The Clinical Pharmacology Core has recently developed and validated a sensitive, multiplex LC-MS/MS assay to measure the intracellular metabolites of the 3 most commonly prescribed NRTIs (tenofovir, emtricitabine, and lamivudine) in dried blood spots collected from patients on therapy. This assay allows investigators to monitor adherence in clinical trials with the convenience of dried blood spot sampling, storage and shipping. The addition of lamivudine, which is generically available, opens up opportunity for international investigations. Our methods and findings are in press in the Journal of Pharmaceutical and Biomedical Analysis (Schauer et al. Validation of an LC-MS/MS Assay to Simultaneously Monitor the Intracellular Active Metabolites of Tenofovir, Emtricitabine, and Lamivudine in Dried Blood Spots. JPBA. In press).

Angela Kashuba
Elias Rosen
Nicole White – Research Specialist
White has been an outstanding analytical chemist at UNC since 1994, and she has received awards in recognition of her exceptional service. White oversees analytical methods and standard operating procedures in the laboratory.
The Kashuba laboratory has been supporting academic and pharmaceutical industry fellows since 1997.
The Kashuba laboratory is a place where graduate education is supported and nurtured.
Talisa Kinsale
Graduate Students
Aaron Devanathan, 2017-2022
Assistant Professor, University of Pittsburgh School of Pharmacy
Erin M.B. Scholz, 2016-2021
Project Manager, Nuventra
Micah Willis
Bryan Guzman
Kaitlyn Maffuid
PhD Candidate, UNC Eshelman School of Pharmacy
Nithya Srinivas, 2014-2018
Research Investigator, Incyte Corporation
Melanie Nicol, PhD, 2009-2014
Assistant Professor, University of Minnesota School of Pharmacy
Michael Cohen-Wolkowiez, PhD, 2007-2012
Associate Professor of Pediatrics, Duke University School of Medicine
Naser Rezk, MS, PhD 2002-2007
Mary Peace McRae, PharmD, PhD, 2001-2005
Assistant Professor, Virginia Commonwealth University
Corbin Thompson, PharmD, PhD
Scientific Investigator, ViiV Healthcare
Postdoctoral Fellows
Joseph Mwangi, PhD 2019-2021
Mass Spectrometry Imaging Fellow
John Prybylski, PharmD, PhD 2019-2021
HIV Pharmacology Research Fellow
Daijha J. C. Anderson, PharmD, RPh 2018-2020
GSK Medical Affairs Fellow
Mac Gilliland, PhD 2017-2019
Assistant Professor, Furman University
Kirsten Moody, PharmD 2017-2019
Medical Science Liason, SpringWorks Therapeutics
Robert Pope, PharmD 2016-2018
Katy Garrett, PharmD 2015-2017
Senior Scientist, Merck
Mike Weber, PharmD 2015-2017
Senior Manager, Clinical Research & Development, SpringWorks Therapeutics
Elizabeth Andrews Shearin, PharmD 2014-2016
Junior Clinical Scientist at Janssen Inc.
Christine Trezza, PharmD 2013-2015
Research Scientist, ViiV
Mackenzie Cottrell, PharmD 2012-2015
Research Assistant Professor, UNC Eshelman School of Pharmacy
Tanja Hadzic, PhD, 2012-2014
Manager, Medical Affairs, Salix Pharmaceuticals
Ben Greener, PharmD, 2011-2013
Jessica Adams, PharmD, 2010-2012
Assistant Professor, University of Sciences, Philadelphia
Racheal Kendrick, PharmD, 2009-2011
Pharmacokineticist II, Quintiles, Kansas
Kevin Brown, PharmD, 2007-2010
Pharmacist, UNC Hospitals
Amanda Jenkins, PharmD, 2008-2010
Research Scientist, United Therapeutics
Sunita Paul, MS, 2007-2008
Clinical Research Manager, New York
Kan Lu, PharmD, 2006-2008
Postdoctoral Research Fellow, UCLA
Julie Dumond, PharmD, 2005-2007
Assistant Professor, UNC Eshelman Schoolof Pharmacy
Manoli Vourvahis, PharmD, 2005-2007
Manager, Pfizer, Inc., Clinical Pharmacology, Infectious Disease, New London, CT
Hiba Tappouni, PharmD, 2004-2006
Principal Clinical Research Scientist, Neurosciences CPDM, GlaxoSmithKline, RTP, NC
Ya-Chi Chen, PharmD, 2003-2005
Senior Pharmacologist, Daichi Pharmaceuticals
Rosa Yeh, PharmD, 2003-2005
Director, Pharmacokinetics Laboratory, SeattleCancer Care Alliance, Seattle, WA
Jennifer Park, PharmD, 2002-2004
Clinical Research Program Manager, GlaxoSmithKline
Leslie Davidson, PharmD, 2002-2004
Esam Hamed, MD, 2009-2014
Amanda Corbett, PharmD, 2001-2003
Clinical Assistant Professor, UNC Eshelman Schoolof Pharmacy
Michael Lim, PharmD, 2001-2003
Oncology Medicine Development Centre, GlaxoSmithKline
Sunila Reddy, PharmD, 2000-2002
Senior Manager, Gilead Sciences
Sherene Min, MD, 2000-2003
Director, GlaxoSmithKline
Clinical Assistant Professor of Medicine, UNC-Chapel Hill
Naumann Chaudry, PharmD, 1999-2001
Director, Medical Affairs, Pfizer
Mary Kennedy, PharmD, 1999-2001
Associate Director, Kosair Charities Pediatric Clinical Research Unit at University of Louisville, KY
Karl Gotzkowsky, PharmD, 1998-2000
Director, Product Development, United Therapeutics Corp., RTP, NC
Jodi Weidler, PharmD, 1997-1999
Senior Director, Clinical Research, Monogram Biosciences












