July 17, 2020
 A group of scientists from UNC investigated the effect of nanomicelles of the antiviral drug Resiquimod against malignant lung cancer. Scientists conducted tests on laboratory mice and showed the effectiveness of Resiquimod in comparison with alternative therapies. Their research findings have recently been published in the journal Science Advances.
A group of scientists from UNC investigated the effect of nanomicelles of the antiviral drug Resiquimod against malignant lung cancer. Scientists conducted tests on laboratory mice and showed the effectiveness of Resiquimod in comparison with alternative therapies. Their research findings have recently been published in the journal Science Advances.
Non-Small cell lung cancer (NSCLC) is the most common lung cancer (80 to 85% of lung cancers) and the cause of most cancer deaths worldwide. Postoperative relapse and metastasis cause death in a significant percentage of cases of NSCLC.
The development of tumors is aided by several defense mechanisms against the body’s immune system. Programmed cell death-1 (PD-1) is a receptor that plays a key role in tumor cell tolerance. Cancer cells actively express its related ligand PD-L1, which binds PD-1 and switches-off the attack of T cells on cancer cells. The United States Food and Drug Administration (FDA) has approved the treatment of NSCLC with PD-1 / PD-L1 inhibitors, but this therapy does not benefit most patients with NSCLC.
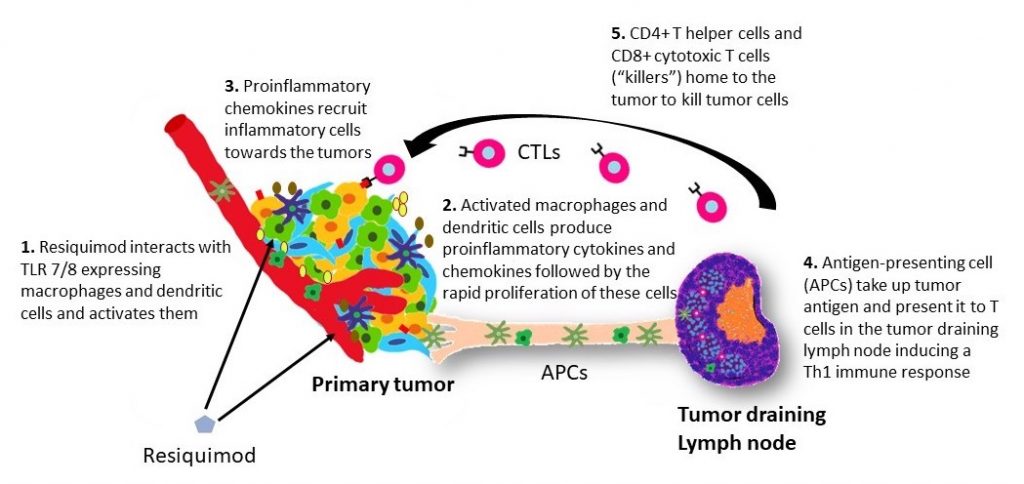
Weakening the defense of tumor cells is possible by another mechanism. Membrane toll-like receptor (TLR) functions as an alarm which activates the immune system and stimulates the production of cytokines that fight pathogen invasion. More than a dozen detected toll-like mammalian receptors, denoted by the abbreviations TLR1 to TLR13, are present in various cells. As for cancer cells, they are recognized as foreign by the protective killer cells of the body. However, in many tumors, up to half the mass is composed of immunosuppressive macrophage cells that suppress the antitumor immune response and thereby promote tumor tolerance; they can be “turned over” by activating their TLR receptors. When you activate the TLRs of tumor macrophages, you can turn them into a pro-inflammatory type of macrophages that in turn engage T cells in to combat the tumor.
To date, only one drug — TLR7 agonist, Imiquimod is used in oncotherapy — administered as a cream for the treatment of superficial basal cell carcinoma and warts. The poor solubility of low molecular weight TLR agonists in water reduces their effectiveness as immunomodulatory drugs. In the form of nanoparticles not exceeding 100 nm, TLR agonists can be targeted to tumors. Scientists at the UNC Eshelman School of Pharmacy and UNC Lineberger Comprehensive Cancer Center synthesized nanomicelles of the drug Resiquimod and tested its antitumor activity in laboratory mice.
“Our system is characterized by maximum simplicity and is based on the principle of polymeric micelles, which we first introduced more than 30 years ago while we still worked in the USSR at the Institute of Applied Molecular Biology and M.V. Lomonosov Moscow State University. Since then several polymeric micelle drugs have been developed and used or evaluated in clinics for the treatment of cancer. The modern technology of polymeric micelles of ultrahigh loading, which we used, although it has not yet been tested in humans, is maximally adapted to this,” said Alexander Kabanov, one of the authors of the study, who is the director of the UNC Center for Nanotechnology in Drug Delivery and professor at both the UNC Eshelman School of Pharmacy and the Faculty of Chemistry of the Moscow State University.
“Resiquimod, a TLR7 and TLR8 agonist, was originally developed as a stimulator of the antiviral immune response. However, the agent was too potent and caused an overreaction of the immune system resulting in flu-like symptoms. Resiquimod was not used for the treatment of viral diseases, owing to its side effects. Nonetheless, we decided to try Resiquimod against NSCLC because this kind of side effect could be possibly mitigated during cancer therapy with numerous agents now available to counteract overshooting immune responses,” says Natasha Vinod, a graduate student at the Joint UNC/NC State Department of Biomedical Engineering and the first author on this paper.
Scientists have shown that Resiquimod reduces tumor growth and significantly increases the lifespan of animals with tumors, which is not observed in the case of the PD-1 inhibitor. The median survival in sick animals treated with Resiquimod was more than double the survival in the group given therapy with a PD-1 inhibitor. Conventional chemotherapeutic drugs are powerless against such tumors.
“Our findings using nanomicelle formulation of Resiquimod performed remarkably well! I’ve evaluated many therapeutic agents in these lung cancer models before, but never before have I seen such impressive therapeutic effects. These effects were seen in a model that does not respond to standard immune checkpoint inhibitors, so this type of approach may give hope to many lung cancer patients needing new options,” said Chad Pecot, a co-author on this publication, who is the Associate Professor in the Division of Oncology and Director of Translational Research at the UNC Lineberger Comprehensive Cancer Center.
Story by Nikolai Kozin, Chemistry Department, Moscow State University.
Latest News

Delesha Carpenter promoted to full professor

Developing new ways to treat heart attacks without surgery


